Water quality plays a vital role in the sterilization of medical devices. Failing to ensure the water used meets the correct purity requirements can lead to adverse patient outcomes, medical device malfunction, and increased costs.
The new ANSI/AAMI ST108:2023 standard addresses this issue by enhancing the recommendations for water used in reprocessing medical devices. Hospital administrators and affected staff should understand this new standard and why it exists.
Failure to do so can not only impact patient safety but can end up costing your facility time and money. FACS is often asked to help investigate potential sources when the Sterile Processing Department (SPD) finds stains or unusual particulates on medical instruments and instrument trays.
This guide will walk you through the key elements of ST108:2023 to help make sure you’re equipped to meet the requirements and protect your patients from the risks of hospital-acquired infection.
Understanding ANSI/AAMI ST108:2023
So, what exactly is ANSI/AAMI ST108:2023, and why is it important?
The new standard sets minimum requirements for the water quality and steam purity used in medical device reprocessing. Medical devices that aren’t properly sterilized due to poor water quality can harbor microorganisms and organic contaminants — thereby creating the risk of infection.
Why was this standard introduced?
Medical instruments today are more complex than ever. With advancements like robotic instrumentation and flexible endoscopes, traditional cleaning methods aren’t always sufficient. Studies show that certain contaminants are nearly impossible to remove from these devices. Add to that the rise of “superbugs” that may resist traditional cleaning measures, and you have a real challenge.
ST108:2023 replaces and expands on the previous AAMI TIR34:2017 guidelines. It addresses emerging issues and ensures that water quality is consistently monitored, validated, and maintained at all times during the device reprocessing cycle.
Compliance Requirements – What You Need to Know
Here is the information you need to make sure your organization is in alignment with this new standard. The requirements are designed to ensure your facility is capable of maintaining the necessary water quality.
1. The Water Management Team (WMT)
One of the key requirements of ST108:2023 and ASHRAE Standard 188 is the formation of a Water Management Team (WMT). This multidisciplinary team is responsible for overseeing all aspects of water quality management within your facility. The team should include representatives from infection control, facilities, engineering, biomedical staff, and third-party expert consultants.
The WMT isn’t just a meet-once-and-forget-about-it group. They are tasked with developing, implementing, and monitoring your sterile processing facility’s water quality management program. It’s crucial that the WMT document all processes and outcomes, ensuring that everyone is on the same page and that the program is continuously updated.
2. Categories of Water Quality
Not all water is created equal, especially when it comes to reprocessing medical instruments. ST108:2023 outlines three distinct categories of water:
- Utility Water: This is the city-supplied water, which may need additional treatment depending on its use. It’s typically used for flushing, washing, and rinsing medical devices. Think of it as the first stage of water quality — it gets the bulk of the contaminants off but might still leave behind impurities expected to be found in drinking water.
- Critical Water: This water is extensively treated to remove any microorganisms, inorganic, or organic materials. It’s used for the final rinse of medical devices and, in some applications, for generating steam. This is where precision matters most — Critical Water ensures that no contaminants are left behind on devices that will come into direct contact with patients.
- Steam: Water that is heated to a vapor phase, either by a central hospital boiler or sterilizer steam generator. It’s essential that the steam used for sterilization meets the specified purity standards, or you risk damaging instruments or reintroducing contaminants during the sterilization process.
Each of these water categories plays a different role in the medical instrument reprocessing cycle, and the standard outlines specific quality benchmarks that must be met for each type.
3. Water Quality Monitoring
The standard calls for an initial Performance Qualification, routine monitoring, ongoing testing, and validation as necessary to maintain compliance.
- Daily, Monthly, and Quarterly Testing: The frequency of testing depends on the type of water. For example, Critical Water might require daily checks on conductivity or pH levels, while Utility Water may only need quarterly testing for hardness. This routine monitoring ensures that any deviations in water quality are caught and corrected before they become a problem.
- Performance Qualification Standards: When a new water treatment system is installed it needs to be tested extensively to ensure it meets the standard’s criteria. This includes daily sampling at critical points in the system. Once the system is proven to meet requirements consistently, you can reduce the frequency of testing but must remain vigilant for any changes.
Monitoring is an ongoing process that ensures your water remains within the parameters set by ST108:2023. This is where many hospitals and other medical facilities can fall short — because the process is complex and time-consuming, it’s easy to let monitoring slip through the cracks.
Section 3: Risk Assessment and Implementation
One of the more complex but crucial aspects of complying with ANSI/AAMI ST108:2023 is the need to conduct a thorough Risk Assessment. This is where you identify potential weaknesses in your facility’s water quality systems and take proactive steps to address them.
Risk analysis is an ongoing evaluation of the water systems in your hospital. Before installing a new water treatment system, the first step is to identify any potential risks to water quality. For example, are there any “dead legs” in your water distribution system where water stagnates, potentially fostering bacterial growth? Are there low-use outlets that might need increased monitoring?
The ANSI/AAMI ST108 standard encourages facilities to look closely at these risk factors and establish a tailored approach. If you’ve never conducted a risk analysis or performance qualification, now is the time to start. By thoroughly understanding where your risks lie, you can implement a monitoring and maintenance plan that fits your facility’s specific needs.
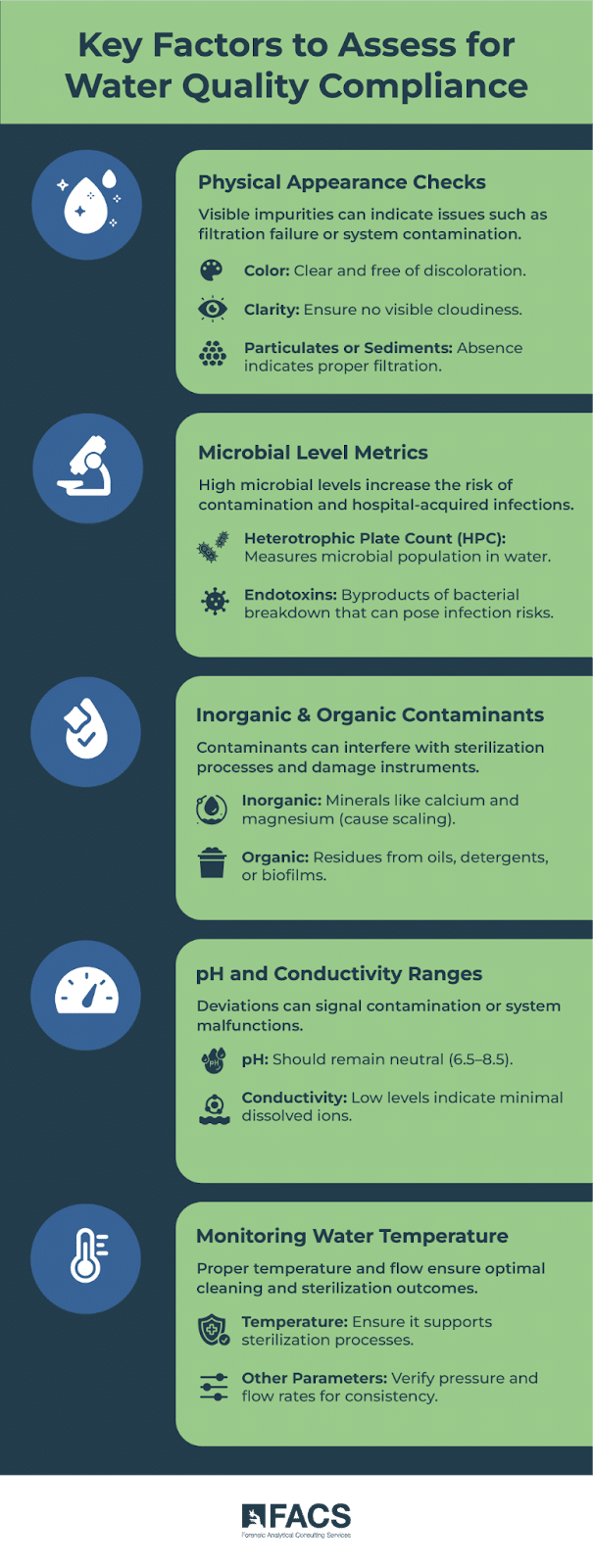
Key factors to assess include:
- Physical appearance of water (color, clarity, and any particulates or sediment)
- Microbial levels, such as heterotrophic plate count (HPC) and endotoxin levels
- Inorganic and organic contaminants that may affect the quality of the water
- pH and conductivity to ensure the water remains within the acceptable range\
- Water temperature and other physical parameters
The risk analysis is completed before any new system installation and reviewed regularly to ensure that any changes to the system, water source, or usage patterns are accounted for.
Implementing Monitoring Systems
Once the risk analysis is complete, the next step is to implement a monitoring program tailored to your facility’s unique needs. This is where the Water Management Team (WMT) determines where and how often samples should be collected.
Key sampling locations include:
- Incoming water supply (to establish baseline quality)
- After each treatment step (to ensure effective filtration or purification)
- At the point-of-use for utility water (e.g., delivery points to washers or sinks) and critical water (e.g., points where water will directly contact medical devices)
- Steam condensate at the sterilizers, to verify the quality of the steam used for sterilization
It’s important to remember that sample collection should not only be scheduled but should also be responsive to changes. For example, if there’s a nearby construction project that could affect your water supply, or if your facility undergoes renovations, you’ll need to adjust your sampling and testing accordingly.
The Role of Continuous Improvement in Staying Compliant
The ANSI/AAMI ST108:2023 standard is a comprehensive guide, but even with the best systems in place, continuous monitoring, and quality improvement are essential to staying compliant. This is where a well-structured Continuous Quality Improvement (CQI) program comes in.
Why Continuous Quality Improvement (CQI) Matters
CQI ensures that your water quality management isn’t static. Water quality can fluctuate due to factors like seasonal changes in the municipal supply, site-specific events (e.g., construction), or the aging of internal plumbing systems. A CQI program helps you stay ahead of these issues by consistently tracking performance, documenting outcomes, and making adjustments as needed.
ST108:2023 places a strong emphasis on integrating CQI into your overall quality management process to ensure every part of the device reprocessing cycle is functioning optimally and that your facility is meeting all regulatory requirements. Staff from engineering, sterile processing, infection control, and other departments should collaborate to track and analyze water quality data.
Here’s How FACS Can Help With Water Quality
Implementing a robust monitoring system and CQI program can be challenging, especially when you’re juggling the many other responsibilities of hospital administration.
FACS can help.
From Risk Analysis to setting up routine monitoring and troubleshooting to identify any water quality issues, FACS experts can work alongside your staff every step of the way. Water quality maintenance is a necessary part of delivering excellent patient care. Call FACS for more information.
Contact FACS by telephone: (888) 711-9998
Contact FACS online: Ask FACS